Recently, the European Chemicals Agency (ECHA) officially announced the inclusion of diphenyl(2,4,6-trimethyl benzoyl)phosphine oxide in the 29th batch of Substances of Very High Concern (SVHC) Candidate List. This brings the number of substances on the SVHC Candidate List to 235.
For the chemicals on the list, the companies concerned have the responsibility to manage the risk of the chemicals and provide information to customers and consumers on the safe use of these chemicals. These substances may be added to the official list in the future. If a substance is on this list, its use will be banned, unless the company applies and the European Commission authorizes them to continue using it.
Specific information on the added substances is as follows:
Substance: Diphenyl(2,4,6-trimethylbenzoyl)phosphine oxide
Alias: Photoinitiator TPO
EC No.: 278-355-8 CAS
No.: 75980-60-8
Reason for addition: Reproductive toxicity (Art. 57(c))
Common Uses: Used in inks and toners, coating products, photochemical polymers, adhesives, sealants and fillers, gypsum modeling clays, etc.
01| The road to domestic development of light curing
Photocuring (photocuring) is the light-induced curing of monomeric, oligomeric, or polymeric substrates and is generally used in film formation processes. The technology has the characteristics of high efficiency, wide adaptability, economy, energy saving, and environmental protection.
Light curing is divided into traditional mercury lamps and emerging UV LED curing. Because the conventional mercury lamp is not used without proper treatment will lead to severe environmental pollution; and UV LED curing with more energy efficient, can be switched on and off at any time, the volume is smaller, and many other advantages are gradually replacing the traditional mercury lamp curing, become the mainstream of light curing equipment.
The proportion of photoinitiators in the light curing formula is low, usually about 2%-5%, but it is pivotal. This is because the light curing reaction takes place through the absorption of UV light by the photoinitiator to produce free radicals, which trigger the polymerization reaction and make the final curing of the product.
Traditional photoinitiators, such as 1173, 184, etc., are a maximum absorption wavelength of UVC short wavelength, so conventional mercury lamp curing is more suitable.
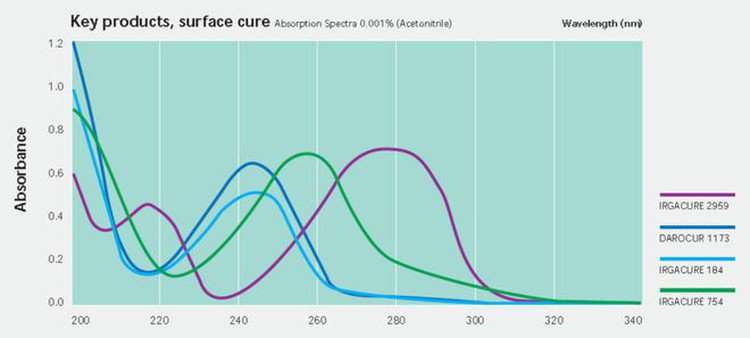
UV LEDs are mainly concentrated in a few wavelengths, such as 365nm, 385nm, 395nm, and 405nm, and the absorption of phosphine oxide photoinitiators in these wavelengths is relatively strong, so they are widely used in UV LED applications.
One of the most representative photoinitiators is TPO, which has high initiation efficiency, no yellowing, and a relatively moderate price. However, due to the booming of UV LED curing in the past few years, the global supply of TPO was tight and hard to find, with the highest unit price exceeding RMB300/kg.
In recent years, due to the continuous expansion of domestic mainstream photoinitiator manufacturers and the entry of new manufacturers, the tight supply of TPO has been greatly alleviated, and the price has fallen back to around RMB 100.
02|TPO toxicity classification and restrictions on use
Photoinitiators are usually small organic compounds, and in cases of incomplete light, these photoinitiator molecules can remain in the cured product, forming potentially migrating substances. In addition, in most cases, the process of free radical generation from photoinitiators is through cleavage. These radicals may form minor molecular weight compounds after their final quenching. These small molecule products pose migration problems and may produce some toxic substances.
With the widespread use of the photoinitiator TPO, its regulation has intensified. According to the EU CLP (Classification, Labelling, and Packaging) regulations, TPO was initially classified as a Class 2 (H361) reproductive toxicant, i.e., "Suspected human reproductive toxicant."
In June 2020, Sweden, a Nordic country, proposed to change the classification to 1B (H360DF) and add it as a skin irritant (H317). This is based on a large body of evidence from animal studies. (1B means "Presumed human reproductive toxicant")
03|Options for alternatives to TPO
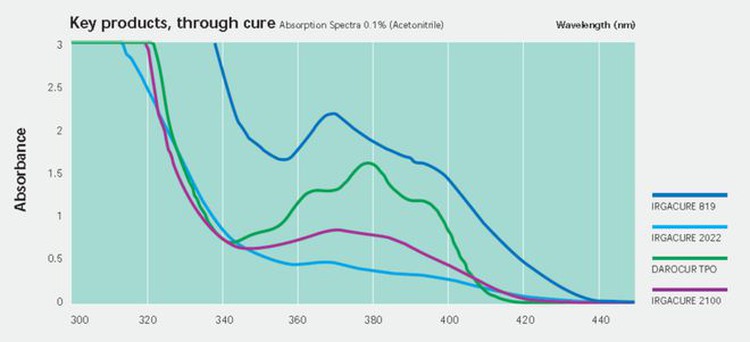
In addition to TPO, there are two other commonly used photoinitiators, TPO-L and 819 (BAPO), which are phosphine oxide photoinitiators with good absorption capacity in the UVA band.

TPO-L has a similar structure to TPO but is less toxic due to substituting a benzene ring for an ethoxy group in the molecule. On the downside, however, TPO-L has a much lower initiation efficiency than TPO.
Another phosphine oxide photoinitiator is 819 (BABO), which can be regarded as TPO with one benzene ring replaced by 2,4,6-trimethyl benzoyl, i.e., with two 2,4,6-trimethyl benzoyl groups. 819 has a higher initiation efficiency than TPO but suffers a more severe yellowing problem. This is not possible where color is required.
In other words, TPO-L and 819 can only replace TPO in some applications, but only partially.
04|The new alternative to TPO - TMO
TMO is (2,4,6-trimethyl benzoyl)bis(p-tolyl)phosphine oxide, CAS 270586-78-2. From a structural point of view, TMO is based on TPO with the introduction of a methyl group on each of the two benzene rings, significantly reducing TPO's biological toxicity.

It has been found experimentally that TMO has an even slightly better initiation efficiency than TPO while not yellowing and migrating much less.

Double bond conversion curves of TMO and TPO initiated TMPTA
Consolidated from:
Chemical New Materials, EU Chemicals Agency, GTS Global Testing, Light Curing New Materials, China Chemical Information Weekly, DT New Materials


2022-08-03


2025-01-06

